Jim Polarine - Technical Services Senior Manager, Beth Kroeger - Technical Services Senior Manager, and Joe McCall - Technical Service Specialist
Myths and misunderstanding abound when it comes to microbiology, from the “5-second rule” for eating food that’s been dropped on the floor to generational lore about starving a cold and feeding a fever. When it comes to cleanroom and pharmaceutical microbiology, some “myths” have become engrained and even endorsed by regulatory bodies. For example, there are still some authorities who insist on rotation of disinfectants to prevent development of resistant bacterial strains. The authors aim here is to use logic and sound science to dispel some Microbiological Myths related to Disinfectant Cleaning of Controlled Environments.
Some of the questionable beliefs, practices and policies we’ve come across are listed below:
MYTH: Environmental Monitoring (EM) data is a reliable predictor of risk to product quality.
FACT: Viable microbial EM data generally reflects a snapshot in time, representing a transient condition that may or may not persist. A single data point (i.e., one surface sample) can not necessarily be extrapolated as relevant beyond its immediate sample parameters. The authors have witnessed contaminated Aseptic Process Simulations (Media Fills) where the associated EM was entirely clean (0 CFU). Conversely, we’ve seen passing results for a Media Fill where the associated EM data showed aberrantly high contamination, multiple Alert & Action level excursions. Interpretation of cleanroom EM data is a highly nuanced practice, best conducted by well trained, educated, and experienced staff.
MYTH: Residue(s) from dried disinfectants on surfaces are inherently risky to drug product quality.
FACT: Any surface residue, under the right conditions, may pose a risk to quality. The theory that disinfectant residues may harbor viable microorganisms is an un-proven idea, and is rather counterintuitive when considering the metabolic needs of viable microorganisms. A risk- and science-based approach to addressing disinfectant residue, or any other cleanroom residue, is the only advisable course of action.
MYTH: Spraying disinfectants onto surfaces achieves superior coverage, and thus effectiveness, compared to mopping or wiping. FACT: Technique matters! Liquid chemical disinfectants work through direct contact with cell membranes. Spraying can be effective if contiguous coverage is achieved. The mechanical action of wiping and mopping has added benefits as well. Comparing the two methods is not a simple or straightforward exercise. Companies must consider many factors in determining the optimal mode of application for their processes and facilities, including but not limited to: surface materials and complexity, size and accessibility of areas, and historical EM results. A formal Risk Assessment can help determine the best approach.
MYTH: EM recoveries of mold from a surface require a Corrective Action using a sporicidal agent on that surface.
FACT: As noted above, EM results are a snapshot in time and typically reflect transient conditions. Given the separation of time between collection of the sample and receipt of the results (typically 3-5 days), many activities have likely occurred in the area that render it is illogical to react as if the conditions stayed the same. Often the surface in question will have already been treated by a germicidal detergent that has fungicidal effectiveness. Prevention is better than reaction; a thorough investigation into the source of the mold, and preventing its recurrence, is a better and more scientifically sound approach.
Myth: More is Better
On numerous visits to bio-pharmaceutical and medical device facilities, the authors have seen complex regimens in place for disinfecting cleanroom surfaces, involving 3 or more varieties of disinfectants and multiple types of sporicidal agents. As an example, a number of companies we’ve recently visited in South America are deploying such complex schemes. These complicated approaches to cleanroom disinfection make it burdensome for the employees performing the tasks, without added benefit in terms of bioburden control. The fact is, routine use of a single broad-spectrum germicidal detergent, coupled with periodic and targeted use of a sporicidal agent, is a fully effective program to achieve sufficient bioburden control in the vast majority of cleanroom environments1.
Q: If a sporicide will kill everything, shouldn’t we just use that all the time?
A: Chemical inactivation of bacterial endospores requires fairly aggressive formulations, such as sodium hypochlorite or hydrogen peroxide PAA blends (peracetic/acetic acid) blends. Using an oxidizer-based sporicide too frequently can cause corrosion issues in the cleanroom as well as EH&S exposure concerns. Therefore, sporicides should be used judiciously, the frequency based on risk and historical EM recoveries of endospore-forming bacteria.
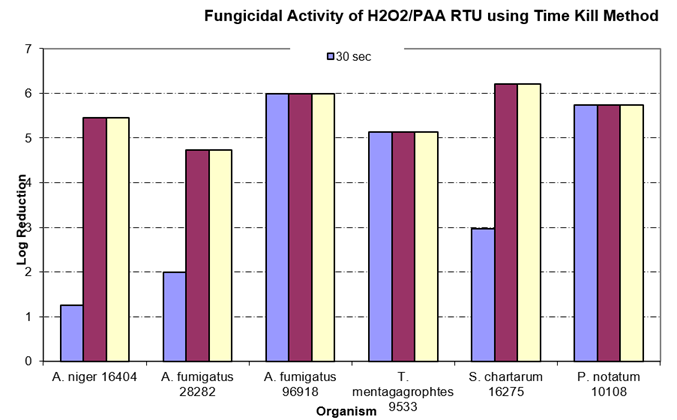
Alcohol is another cleanroom agent which is not always used correctly. Many facilities use only 70% IPA on items that will be passed into the cleanroom environment, assuming it is effective vs. fungal and bacterial spores. There may be some effect on fungal spores as seen in the table below (Time-Kill study2), but a true sporicidal agent is required to prevent ingress of spore-formers into the cleanroom
H2O2 / PAA RTU Efficacy Against Molds
Often operators are trained per SOP to use unidirectional overlapping stokes with flat head mops on walls and floors…however this technique can devolve if training was insufficient and practices are not enforced. Making up correct use-dilutions is another key element for effective disinfection3. Graduated cylinders and unit dose packets are the most accurate way. Pouring concentrated disinfectant into a bucket using the “eyeball” method is never advisable.
An effective cleaning and disinfection program should always have a disinfectant with broad-spectrum effectiveness and a sporicide to address bacterial endospores in cleanroom operations.
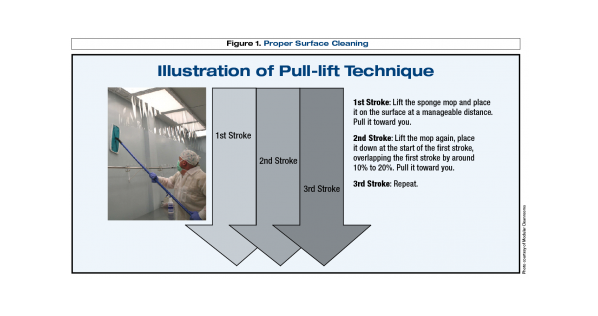
(Image Credit: http://www.pppmag.com/article/714/June_2010/Cleaning_Practices_for_Cleanroom_Contamination_Control/)
Myth: Disinfectant residues will harbor bioburden and interfere with a sporicide’s efficacy
Routine application of disinfectant and sporicidal agents is required to maintain environmental control of cleanrooms. Routine use of these products may leave behind surface residues. Issues may occur due to use of incompatible chemistries in rotation, but does disinfectant residue alone promote microbial contamination? And do disinfectant residues interfere with sporicidal efficacy?
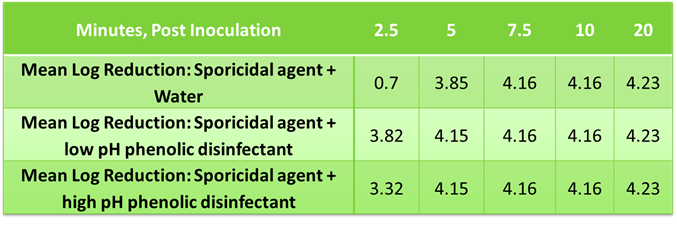
STERIS’s next generation phenolic disinfectants were tested as residues in a time-kill study to assess impact on the efficacy of a sporicidal agent (SporKlenz® RTU) vs. Bacillus subtilis, ATCC# 19659. The solids (µg/cm2) from dried disinfectant were determined in a separate study where the amount of residue was calculated after several applications to a 116 cm2 stainless steel test coupon to mimic typical use in the cleanroom as well as the amount of SporKlenz RTU to cover a surface in typical applications as the diluent. In the case of the later, 120 mgs of SporKlenz RTU sterilant was determined to cover a 116 cm2 coupon. The ratios determined in this experiment were extrapolated to determine dilution of disinfectant residue to spike into a solution of sporicidal agent.
A volume of 0.1 mL of the microorganism suspension was added to 9.9 mL of the test solution (i.e., Spor-Klenz RTU sterilant spiked with either a low pH or high pH phenolic disinfectant). A buffer control was spiked using the same amount. Post inoculation, a 0.1 mL sample was removed at 2.5, 5, 7.5, 10, and 20 minutes. The samples were neutralized, plated, and incubated for 48-49 hours at 37±2°C. The viable organisms per mL of sample were determined by standard aerobic plate count.
Mean Log Reduction of B. subtilis using phenolic residues and sporicidal agent solution
Similarly, dried disinfectant residues were evaluated to determine impact to sporicidal efficacy since this reflects typical cleanroom practices. Samples were prepped to represent 20 days of disinfectant application to a known area, allowed to dry, then treated with a volume of sporicidal agent sufficient to cover the known area or 8.6 µL/cm2. The inoculum was prepared from a spore suspension of Bacillus subtilis, ATCC# 19659. The test spore suspension was diluted with DI water to approximately 3.0 x 108 CFU/mL. The suspension was added to tubes containing test samples or Butterfields buffer to give a 1% v/v mixture of microorganism in test product or buffer. At various timepoints, samples were neutralized, plated, and incubated for 48 – 50 hours at 37 ±2°C post inoculum. The viable organisms per mL of sample were determined by standard aerobic plate count and transformed to log 10 values for analysis.
Mean Log Reduction of B. subtilis using dried phenolic residues and sporicidal agent solution
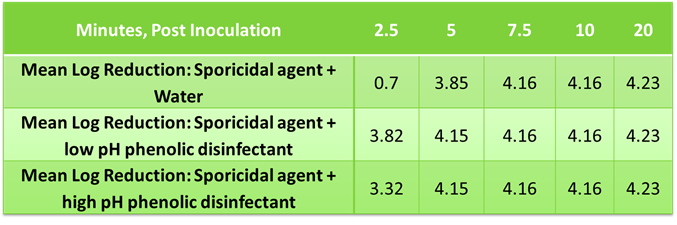
The data shows no inhibitory / antagonistic effects of the dried residue with sporicidal effectiveness.
Basic Training
It is a regrettable fact in the Bio-Pharma, OSD, and Medical Device industries that the “cleaning crews”, those responsible for disinfectant cleaning of regulated cleanroom production areas, often receive the least amount of training, guidance, and oversight compared to other departments (e.g. QC, Mfg.). Many times, in the spirit of cost savings, the role of disinfectant cleaning is relegated to a contract cleaning company, further complicating training issues and removing the cleaning crew from QC and QA oversight. Off-shift hours further obscure what’s happening on the shop floor. In today’s global market, there can be language barriers to deal with as well. To utterly complicate matters, successful disinfection of cleanroom surfaces and environments is wholly dependent on operator skills and technique. Unlike physical cleaning of visible soil, cleanroom surfaces typically already appear clean. The employees then are tasked with removing invisible matter, whether viable bioburden or sub-visible particulate contamination. There are little to no visual cues as to when a surface has been effectively decontaminated. It is for these reasons that the operators who perform disinfectant cleaning must be exceptionally well trained and versed in principles of microbiology, contamination control, and aseptic practices.
Would your Cleaning Crew recognize and react to the conditions below?

Misaligned ceiling tiles, exposing cleanroom to exterior contaminants

Damaged primary surfaces: gouges and gaps in flooring result in difficult to clean areas.

Poorly sealed seams between panels, leading to trapped moisture and mold proliferation.
Additional information regarding disinfectant cleaning in controlled environments can be found at:
sterislifesciences.com/resources/documents/technical-tips/cleaning-process-design-stage
sterislifesciences.com/education-and-training/cleanroom-standards
sterislifesciences.com/education-and-training/cleaning-and-cleaning-validation-seminar
sterislifesciences.com/education-and-training/microbial-control-in-pharmaceutical-and-medical-device-cleanrooms
References:
- PDA Technical Report No. 70 (2015). Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities. Available from Parenteral Drug Association, Inc.
- Polarine, J., Macauley, J., Karanja, P., Klein, D., Martin, A. (2009) Evaluating the Activity of Disinfectants Against Fungi. Cleanrooms: The Magazine of Contamination Control Technology 23(2).
- http://www.pppmag.com/article/714/June_2010/Cleaning_Practices_for_Cleanroom_Contamination_Control/